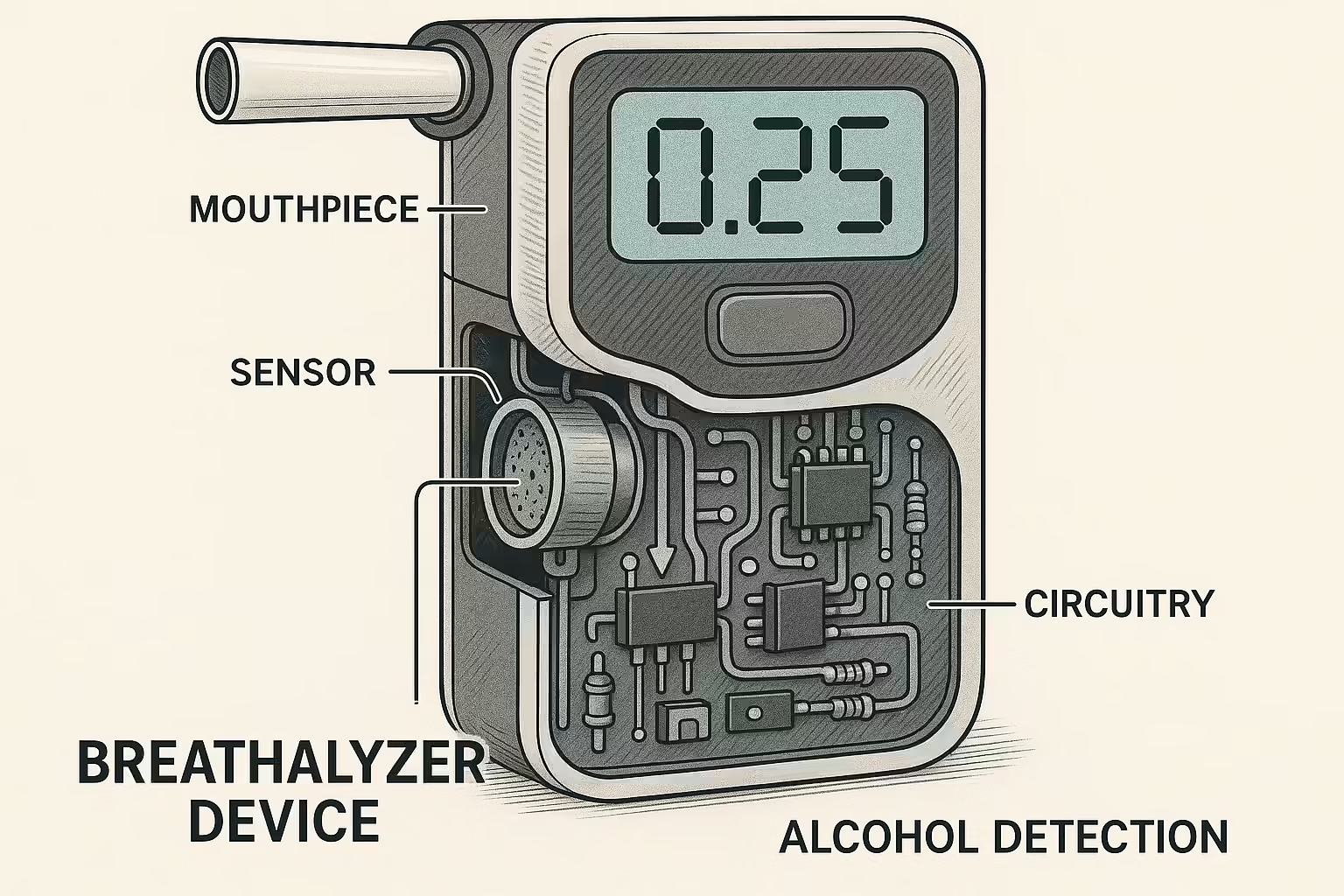
A breathalyzer estimates blood alcohol content (BAC) by measuring ethanol molecules in exhaled breath using three main technologies: fuel cell sensors (most accurate), semiconductor sensors (most affordable), and infrared spectroscopy (most sophisticated). The device works on Henry’s Law, establishing a 2100:1 ratio between breath and blood alcohol concentrations, converting breath alcohol measurements into reliable BAC readings that reflect bloodstream alcohol levels.
When you consume alcohol, it is absorbed into your bloodstream and travels to your lungs, where some evaporates into air sacs (alveoli). As you exhale, this alcohol-containing air is analyzed by sophisticated sensors that detect ethanol molecules with remarkable precision. Modern breathalyzers represent decades of scientific advancement, combining fundamental physics principles with cutting-edge sensor technology to provide law enforcement, employers, and individuals with accurate alcohol detection capabilities.
How Does Alcohol Move from Blood to Breath?
The Journey from Consumption to Detection
When you consume alcohol, it doesn’t stay in your stomach. Within minutes, ethanol molecules begin to be absorbed through your gut and intestinal walls directly into your bloodstream. This alcohol-rich blood then circulates throughout your entire body, including your lungs, where the process of breath testing begins. As blood flows through the tiny capillaries surrounding your lung’s air sacs (alveoli), something fascinating happens. Alcohol molecules, being naturally volatile, evaporate from the blood into the alveolar air. This process occurs automatically due to alcohol’s chemical properties-it wants to escape from liquid into gas form.
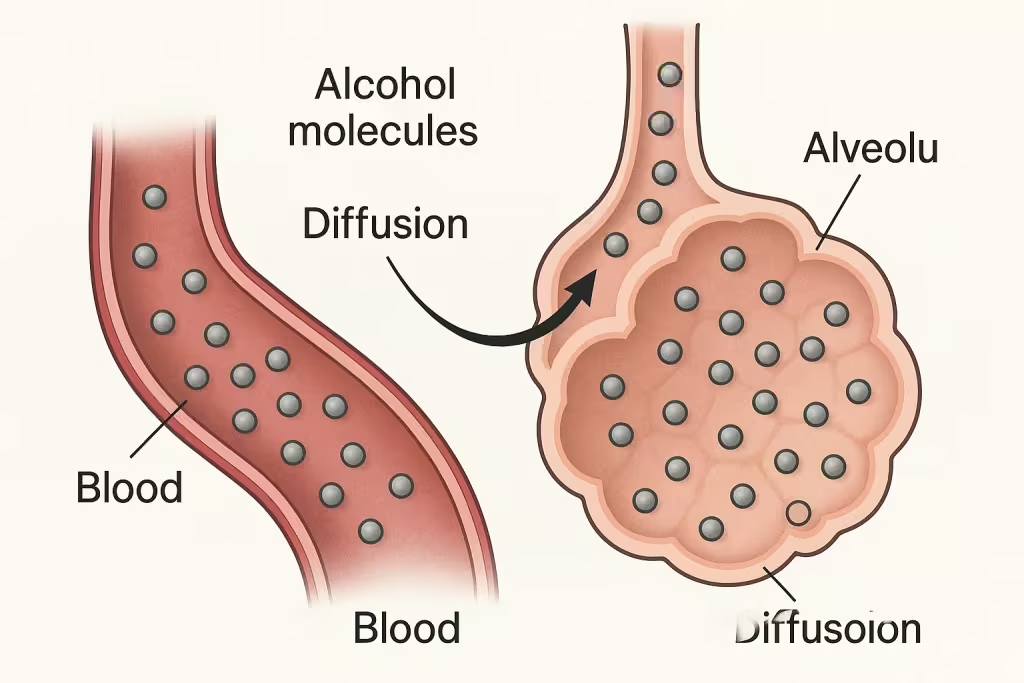
Scientific diagram illustrating how alcohol moves from blood to lung air through alveoli
As blood flows through the tiny capillaries surrounding your lung’s air sacs (alveoli), something fascinating happens. Alcohol molecules, being naturally volatile, evaporate from the blood into the alveolar air. This process occurs automatically due to alcohol’s chemical properties – it wants to escape from liquid into gas form.
Henry’s Law: The Scientific Foundation
The entire breathalyzer industry rests on a 19th-century scientific principle called Henry’s Law. This law describes how gases dissolve in liquids and, more importantly for our purposes, how they escape from liquids into gas form. Henry’s Law establishes that at a given temperature, there’s a predictable relationship between the concentration of a volatile substance in liquid (your blood) and its concentration in the gas above that liquid (your breath).
For alcohol specifically, scientists have determined this relationship follows a 2100:1 partition ratio. This means that 2,100 milliliters of deep lung air contain approximately the same amount of alcohol as 1 milliliter of blood. However, this ratio isn’t universal – it can vary between individuals from 1,500:1 to 3,000:1 depending on factors like age, genetics, gender, and even your current level of intoxication.
Why Deep Lung Air Matters for Accuracy
Not all exhaled air is created equal when it comes to breathalyzer testing. The first air you exhale comes from your mouth and upper respiratory tract, which may contain residual alcohol from recent drinking, mouthwash, or even burping. This “mouth alcohol” can artificially inflate readings and doesn’t reflect your actual blood alcohol level.
The crucial factor in breathalyzer testing is the source of the exhaled air. The first air you exhale, from your mouth and upper respiratory tract, may contain residual alcohol, which can lead to inflated readings. The accurate source of air is from deep in your lungs, specifically from the alveolar sacs where gas exchange occurs. This alveolar air, having equilibrated with your blood at body temperature (98.6°F), provides the most accurate correlation to your actual blood alcohol concentration.
Modern breathalyzers are equipped with slope detection algorithms, a crucial feature that ensures accurate readings. These systems monitor your breath alcohol concentration as you exhale, looking for the characteristic pattern where alcohol levels rise rapidly initially, then plateau as the deep lung air is expelled. This pattern is a key indicator of the source of the air, ensuring that the breathalyzer is analyzing the right air for an accurate reading.
The Three Detection Technologies Explained
How Do Fuel Cell Breathalyzers Work?
Fuel cell technology represents the gold standard in breath alcohol testing, used by law enforcement agencies worldwide for its superior accuracy and reliability. These devices employ an elegant electrochemical process that targets ethanol molecules explicitly.
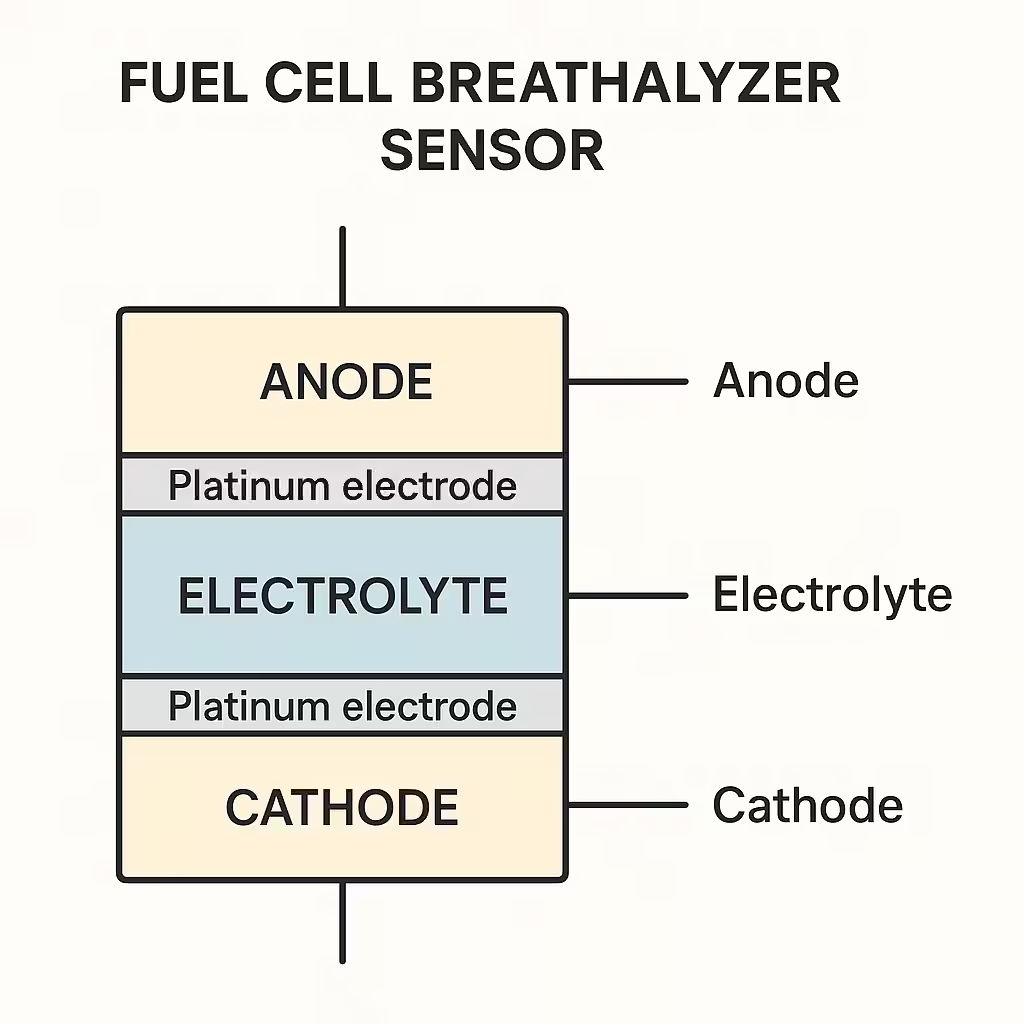
Technical diagram of fuel cell breathalyzer showing platinum electrodes and electrochemical process
At the heart of a fuel cell breathalyzer are two platinum electrodes separated by a porous acid-electrolyte material. When your alcohol-containing breath passes through this fuel cell, something remarkable happens at the molecular level. The platinum acts as a catalyst, causing the ethanol to oxidize (combine with oxygen) and break down into acetic acid, protons, and electrons.
The electrons produced by this reaction flow through a wire circuit, creating an electrical current that’s directly proportional to the amount of alcohol in your breath. The more alcohol present, the stronger the current. The breathalyzer’s computer measures this current and converts it into a precise BAC reading.
What makes fuel cell technology superior:
- Exceptional accuracy: Can measure up to 3 decimal places (0.001 BAC)
- Ethanol-specific detection: Only reacts with ethanol, avoiding false positives from substances like acetone or ketones
- Extended lifespan: Can last 5+ years with proper maintenance
- Linear response: Maintains accuracy across the complete alcohol concentration range from 0.000-0.400% BAC
- Less frequent calibration: May require calibration only once yearly or after 1,500 tests
How Do Semiconductor Breathalyzers Work?
Semiconductor breathalyzers take a different approach, using heated tin dioxide (SnO2) sensors that operate at temperatures between 300-400°C. These devices are popular for personal use due to their affordability, though they sacrifice some accuracy for cost savings.
The science behind semiconductor sensors involves a chemical reaction at high temperatures. An internal coil heats a steel mesh film coated with tin dioxide. When alcohol vapor from your breath contacts this heated surface, it causes a chemical reaction that changes the electrical resistance of the tin dioxide material.
The breathalyzer measures this resistance change as a voltage output and converts it to an alcohol concentration reading. The process happens quickly – typically within 2-3 seconds of providing a breath sample.
Semiconductor technology characteristics:
- More affordable: Typically 2-3 times cheaper than fuel cell devices
- Less precise: Usually measures to 2 decimal places with accuracy of ±0.01% BAC
- Interference-prone: May react to cigarette smoke, acetone, ketones, and volatile chemicals
- Shorter lifespan: Typically lasts only a few years
- Frequent calibration needed: Requires calibration every 6-12 months
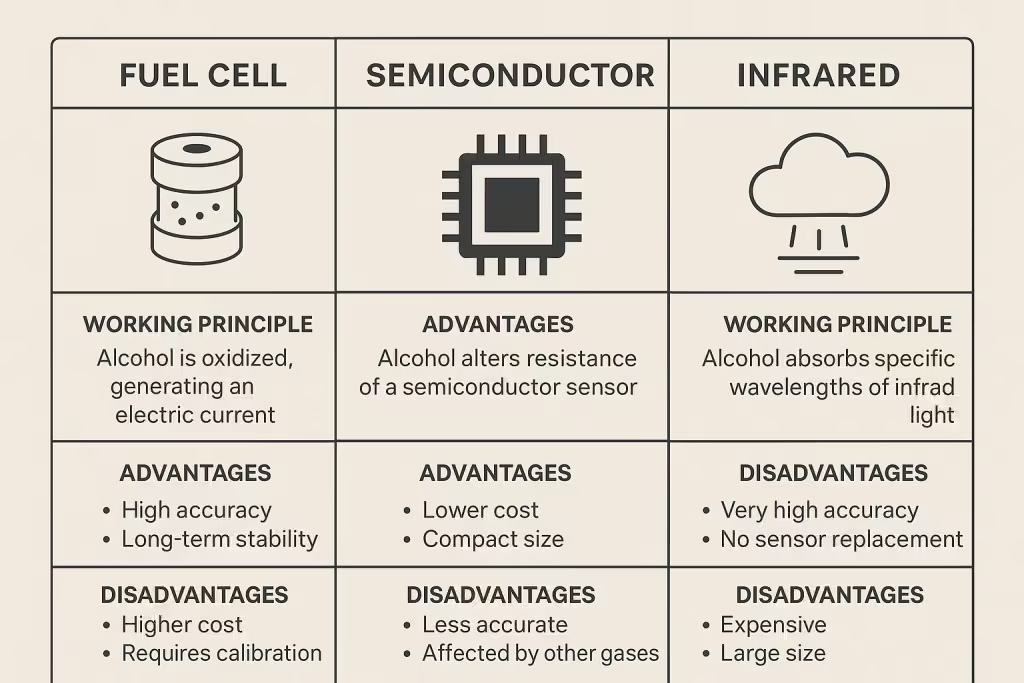
Comparison infographic showing three main breathalyzer technologies and their key features
How Does Infrared Spectroscopy Detect Alcohol?
Infrared spectroscopy represents the most sophisticated breathalyzer technology, primarily used in professional evidential testing equipment that must meet the highest legal standards. This method exploits a fundamental property of molecules – they absorb specific wavelengths of light, making it a reliable technology for accurate readings.
The infrared detection process works like a molecular fingerprint system. An infrared lamp emits light through your breath sample chamber. Ethanol molecules naturally absorb infrared light at specific wavelengths, particularly around 3.39, 3.48, 7.25, 9.18, 9.50, and 11.5 micrometers. A rotating filter wheel allows different wavelengths to pass through to a sensitive detector.
The detector measures how much infrared energy reaches it after passing through your breath sample. The difference between the reference light (no alcohol) and the absorbed light indicates the concentration of alcohol molecules in your breath.
Advanced infrared spectroscopy features:
- Multiple wavelength analysis: Can distinguish between ethanol and interfering substances
- Real-time breath monitoring: Detects mouth alcohol contamination patterns
- Highest precision: Most accurate method available for evidential testing
- Interference detection: Advanced algorithms identify and exclude non-alcohol compounds
- No sensor degradation: Light-based detection doesn’t wear out like chemical sensors
What Can Cause False Positive Breathalyzer Readings?
Understanding what can interfere with breathalyzer accuracy is crucial, as studies suggest that approximately 23% of individuals tested may have actual blood alcohol concentrations significantly different from breathalyzer readings, sometimes by up to 15%. Several factors can lead to inaccurate results.
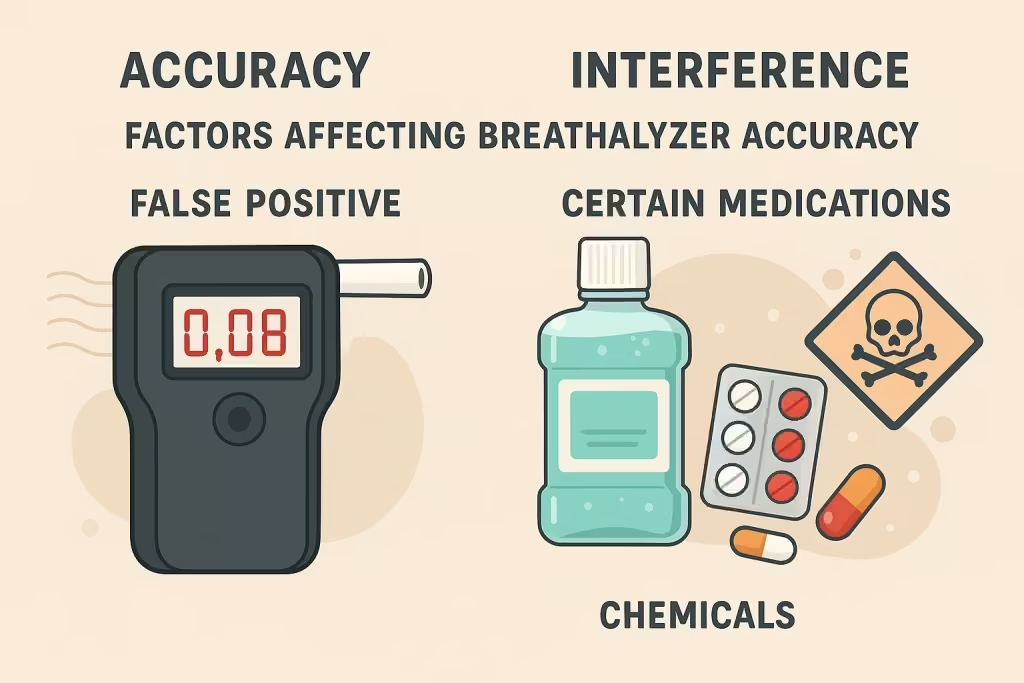
Infographic showing common factors that can cause false positive breathalyzer readings
Physiological Factors That Affect Accuracy
Mouth alcohol contamination represents the most common cause of false positive readings. This occurs when alcohol from sources other than your lungs creates artificially elevated readings. The alcohol doesn’t need to come from drinking – it can originate from:
- Recent alcohol consumption (residual alcohol remains for 15-20 minutes)
- Burping or regurgitation brings stomach alcohol to the mouth
- Gastroesophageal reflux disease (GERD) causes alcohol vapor to rise from the stomach
- Alcohol-containing mouthwash, breath spray, or medications
- Dental appliances or dentures retain alcohol residue
Medical conditions can significantly impact breathalyzer accuracy. People with diabetes may produce acetone through a process called ketosis, especially those following ketogenic diets or experiencing diabetic ketoacidosis. Some breathalyzers, particularly older semiconductor models, cannot distinguish between acetone and ethanol, leading to false positive readings.
Research shows that individuals in ketosis may produce enough acetone to trigger false-positive BAC readings. This is particularly problematic because the symptoms of diabetic ketoacidosis – confusion, disorientation, and unusual breath odors – can mimic alcohol intoxication.
Environmental and Chemical Interference
Workplace chemical exposure presents a significant challenge for accurate breath testing. Industrial solvents, paints, adhesives, gasoline vapors, and cleaning chemicals can all interfere with breathalyzer readings. Workers in auto body shops, manufacturing facilities, or chemical plants may register false positives even without consuming alcohol.
Medications and medical products commonly cause interference issues:
- Asthma inhalers containing albuterol, salmeterol, and budesonide can cause false positives immediately after use, with readings potentially as high as 0.21 BAC
- Cold and flu medications like NyQuil contain alcohol
- Oral gels such as Anbesol are used for tooth pain
- Hand sanitizers and alcohol-based cleaning products
Individual Biological Variations
The 2100:1 partition ratio that breathalyzers rely on isn’t constant across all individuals. Factors affecting this ratio include:
- Body temperature: A 1.8°F increase can affect the partition ratio
- Age and gender: Older individuals and women may have different ratios
- Breathing patterns: Shallow breathing provides less accurate samples
- Time since consumption: BAC can still be rising when tested
Why Is Breathalyzer Calibration Important?
Calibration ensures breathalyzer accuracy by adjusting the device’s response to known alcohol concentrations. Without proper calibration, sensors naturally drift over time, potentially providing readings that are significantly higher or lower than actual blood alcohol levels.
How Often Do Breathalyzers Need Calibration?
Calibration requirements vary significantly based on device type and usage:
- Law enforcement devices: Must be calibrated monthly
- Personal breathalyzers: Every 6-12 months, depending on usage
- High-volume workplace testing: More frequent calibration required
- Professional evidential devices: Some require calibration checks every 7 days
In Texas, breathalyzers must be calibrated according to strict schedules, with most devices requiring calibration checks every 30 days. Missing or incomplete calibration records can provide grounds for challenging breathalyzer evidence in court.
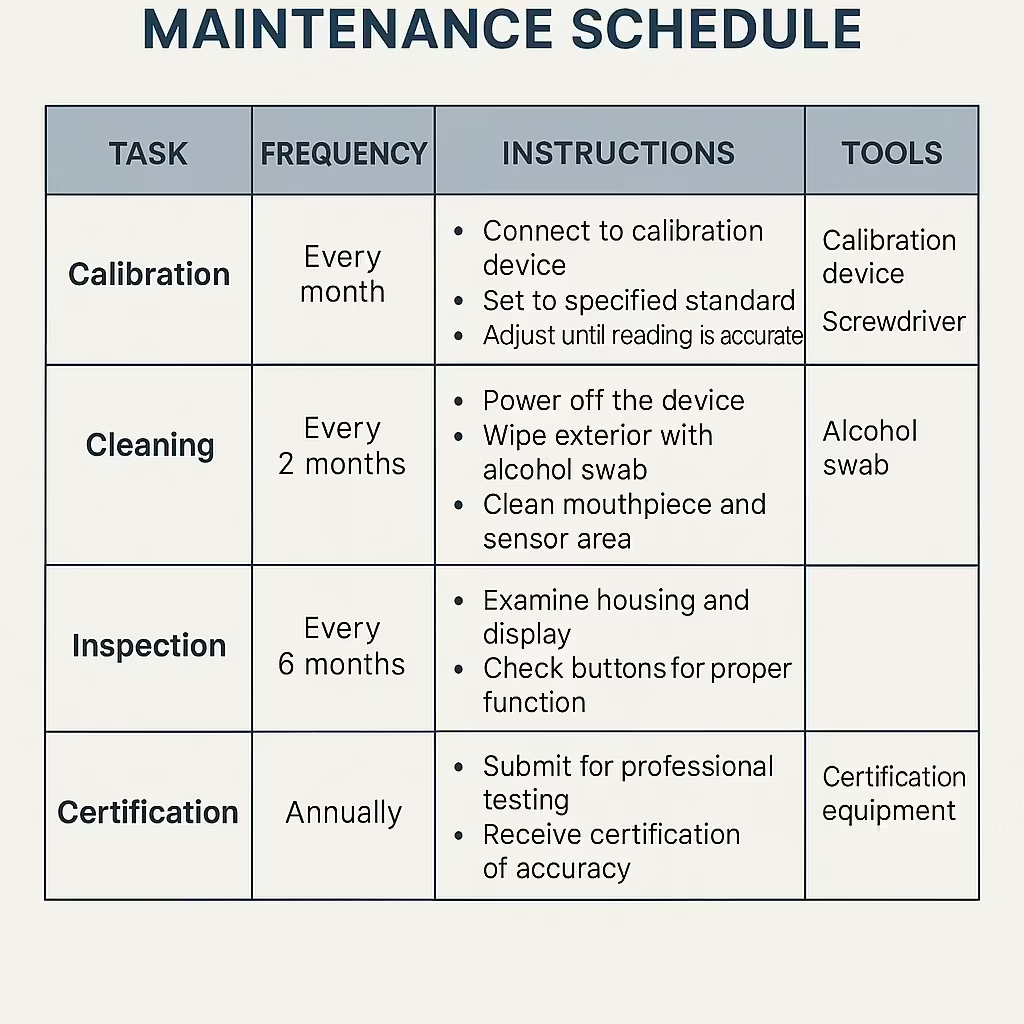
Infographic detailing breathalyzer calibration requirements and maintenance schedules
How Is Breathalyzer Calibration Performed?
Professional calibration involves two primary methods:
Wet bath simulators use heated alcohol solutions at precise temperatures (typically 32°C to match human breath temperature). A known concentration of alcohol in water is heated, and the vapor produced is fed through the breathalyzer. The device should read exactly the known concentration.
Compressed gas standards employ pressurized alcohol-air mixtures with certified concentrations. These systems are more portable and are often used for field calibration of police breathalyzers.
Both methods must be traceable to National Institute of Standards and Technology (NIST) references to ensure legal admissibility. Calibration solutions must come from refrigerated bottles that have been used for less than their specified shelf life.
What Happens When Calibration Fails?
Poor calibration can lead to wildly inaccurate breathalyzer readings. For legal admissibility, evidential breath testers typically must maintain accuracy within ±0.005% BAC at the 0.050% BAC level. If calibration reveals the device is outside this tolerance, it must be repaired and recalibrated before returning to service.
Temperature and environmental factors significantly impact calibration accuracy. Extreme Texas heat can cause sensor drift, battery performance issues, and expansion of internal components, affecting calibration. Rapid temperature changes, such as moving from air-conditioned patrol cars to hot outdoor conditions, can also affect device performance.
Modern Applications Beyond DUI Enforcement
What Are Breathalyzers Used for Besides Traffic Stops?
The global breathalyzer market was valued at USD 2.6 billion in 2023 and is estimated to grow at a 16.8% CAGR through 2032, driven by expanding applications far beyond traditional law enforcement use.
Workplace safety testing represents the fastest-growing segment. Companies implement breathalyzer programs for:
- Safety-sensitive positions: Transportation, construction, manufacturing, and healthcare workers
- Accident investigation protocols: Immediate testing following workplace incidents
- Random screening programs: Deterring alcohol use during work hours
- Pre-shift testing: Ensuring workers are fit for duty
Personal monitoring devices have gained popularity as consumers become more conscious of responsible drinking. Modern personal breathalyzers offer:
- Smartphone connectivity: Apps track BAC trends over time
- Social features: Share results with friends or rideshare apps
- Legal limit warnings: Alerts when approaching 0.08% BAC
Medical and rehabilitation applications utilize breathalyzers for:
- Treatment compliance monitoring: Verifying sobriety during rehabilitation programs
- Medical facility screening: Emergency room patient assessment
- Research applications: Studies on alcohol metabolism and behavior
How Accurate Are Smartphone Breathalyzers?
Research shows smartphone-paired breathalyzers vary widely in accuracy, with some devices dangerously underestimating BAC levels. A comprehensive study published in 2021 found that consumer devices often failed to meet professional accuracy standards, potentially giving users false confidence about their sobriety.
The FDA has not approved most consumer breathalyzer apps for safety-critical decisions. While some smartphone-connected devices use the same fuel cell technology as professional units, many rely on less accurate semiconductor sensors to keep costs down.
Legal Standards and Evidence Requirements
What Makes a Breathalyzer Test Legally Admissible?
For breathalyzer results to be admissible in court, strict protocols must be followed:
Device certification and maintenance:
- Breathalyzer must be on the approved equipment list
- Current calibration certificates must be available
- Maintenance logs must be complete and up-to-date
Operator requirements:
- The officer must be certified on the specific device model
- A proper testing procedure must be followed
- 15-minute observation period required before testing
Quality control measures:
- Air blank tests to verify no ambient contamination
- Duplicate breath samples for consistency
- Chain of custody documentation
What Is the Difference Between Screening and Evidential Devices?
Preliminary Breath Tests (PBTs) used on the roadside are screening devices with less stringent accuracy requirements. These handheld units help officers establish probable cause for arrest, but their results typically aren’t admissible in court.
Evidential Breath Testers (EBTs) are stationary devices meeting strict accuracy standards of ±0.005% BAC. These machines, usually located at police stations, provide results that can be used as evidence in DUI prosecutions.
The distinction matters legally – while a PBT reading of 0.08% might justify an arrest, conviction typically requires confirmation from an evidential device following proper protocols.
Future Technology Trends
What Innovations Are Emerging in Breathalyzer Technology?
Artificial intelligence integration represents the next frontier in breathalyzer development. AI algorithms can:
- Enhance pattern recognition: Better distinguish between mouth alcohol and deep lung air
- Improve interference detection: Identify and compensate for environmental factors
- Predict accuracy: Alert operators to potential reading issues
Wearable technology promises continuous alcohol monitoring without traditional breath samples. Transdermal sensors can detect alcohol through skin, enabling:
- 24/7 monitoring: Continuous BAC tracking for rehabilitation programs
- Smartwatch integration: Discrete monitoring with smartphone alerts
- Predictive warnings: Alerts before reaching dangerous levels
Enhanced connectivity features are transforming breathalyzer capabilities:
- Cloud-based data storage: Centralized monitoring for fleet management
- GPS integration: Location tracking for compliance verification
- Real-time reporting: Instant alerts to supervisors or probation officers
Market Growth and Industry Trends
The breathalyzer market is projected to reach USD 10.9 billion by 2032, growing at a 16.7% CAGR, driven by:
- Stricter DUI regulations globally: More countries implementing lower BAC limits
- Workplace safety requirements: Increased corporate adoption
- Personal safety awareness: Growing consumer market
North America dominates the current market with approximately 44% market share. In comparison, the Asia Pacific region is expected to show the highest growth rate at 17.71% CAGR due to rapid urbanization and increasing road safety concerns.
Key Takeaways:
Breathalyzers work by detecting alcohol molecules in deep lung air using three main technologies: fuel cell sensors (most accurate for professional use), semiconductor sensors (most affordable for personal use), and infrared spectroscopy (most sophisticated for evidential testing).
The fundamental science relies on Henry’s Law, which establishes a predictable 2100:1 ratio between breath and blood alcohol concentrations. However, this ratio can vary significantly between individuals, and accuracy depends heavily on proper calibration, deep lung air sampling, and avoiding contamination from mouth alcohol or interfering substances.
Accuracy concerns remain significant, with studies showing that breathalyzer tests are accurate only approximately 40% of the time, and various factors, including medical conditions, environmental chemicals, and device maintenance, can affect results.
Modern applications extend far beyond DUI enforcement to include workplace safety testing, personal monitoring, and medical rehabilitation programs. The technology continues advancing with AI integration, smartphone connectivity, and wearable devices, though these innovations must balance convenience with the accuracy required for safety-critical decisions.
Understanding breathalyzer limitations is crucial for both users and those subject to testing. While these devices have revolutionized alcohol detection and undoubtedly saved countless lives by deterring drunk driving, their results should be interpreted within the context of their scientific principles, calibration status, and the specific circumstances of each test.
As technology continues evolving, breathalyzers will likely become more accurate, portable, and integrated into our daily lives. However, the fundamental scientific principles – Henry’s Law, proper sampling techniques, and regular calibration – will remain the cornerstone of reliable alcohol detection for years to come.